The given question is incomplete. The complete question is:
10 gram sample of methane
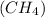 has the same number of molecules as 10 gram sample of
has the same number of molecules as 10 gram sample of
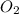 . True or False
. True or False
Answer: Thus the statement is False.
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance contains avogadro's number
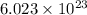 of particles.
of particles.
To calculate the moles, we use the equation:
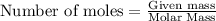
1.
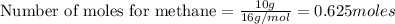
Now 1 mole of
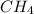 molecule contains =
molecule contains =
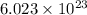 of molecules
of molecules
0.625 mole of
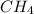 molecule contains =
molecule contains =
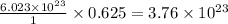 molecules
molecules
2.
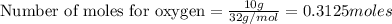
Now 1 mole of
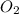 molecule contains =
molecule contains =
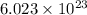 of molecules
of molecules
0.3125 mole of
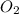 molecule contains =
molecule contains =
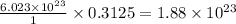 molecules
molecules
The number of molecules are different.