The PH of a solution is defined by the function:
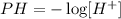
Let's try some values for H+ as follows:
H+={3, 2, 1, 0.1, 0.01, 0.001}
Calculating with any scientific calculator, computer, or math tool, we get the following values for PH:
PH={-0.47, -0.3, 0, 1, 2, 3}
With these values, we plot and graph the function as seen below:
The PH value is 0 when H+=1
The PH value is 1 when H+=0.1