First of all, let's define the concentration of the solution they give us. It tells us that the solution must be 1.3M, this is a measure of molarity that tells us the number of moles of solute (NaNO3) in 1 liter of solution.
So from this information, we can find the number of moles of NaNO3 and from there we can find the mass using the molar mass of NaNO3. Let's proceed to do the calculations, let's first find the necessary moles of NaNO3
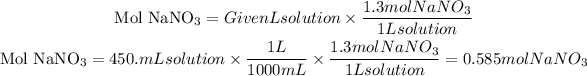
Now, the molar mass of NaNO3 is equal to 84.9947 g/mol, from the number of moles we can find the grams needed:
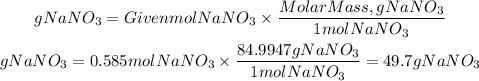
Answer: for making 450.mL of a 1.3 M NaNo3 solution is needed 49.7 g of NaNO3