The Ideal gas law is as follows:

Since we have the informationof how many liters of F₂ we have as well as the temperature and pressure conditions, we can calculate how many moles of F₂ reacted:
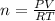
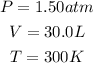
Since ew have the pressure in atm and the volume in L, we can use the following R constant:
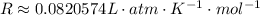
So, we have:

Now, usin the balanced reaction:
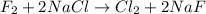
We can see that for each mol of F₂ that reacts, 2 mols of NaCl will react, so:
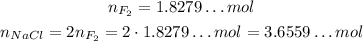
Now, to get this value in mass, we will need the molar mass of NaCl:
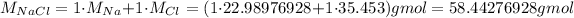
Now, we can calculate the mass of NaCl:
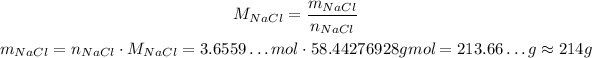
So, approximately 214 g of NaCl wil react.