Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, according to the thermodynamic definition of the Gibbs free energy:
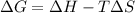
In the scenario by which G>0 (nonspontaneous) it would be possible for reaction to have a G<0 (spontaneous) if:
- If H>0 and S>0, then the temperature should be increased until the process was entropy-driven for it to be possible.
- If H> and S<0, then it'd be impossible to have an spontaneous process.
- If H<0 and S>0 then the process was not spontaneous.
- If H<0 and S<0 it is possible if the temperature is not high enough to ensure the process is enthalpy-driven.
Best regards!