Answer
The percent composition of Cu = 88.82%
The percent composition of O = 11.18%
Step-by-step explanation
Given:
Copper (I) oxide
What to find:
The percent compositions for each of the elements in the compound.
Step-by-step solution:
Step 1: The first step is to write the molecular formula of copper (I) oxide.
The molecular formula of copper (I) oxide is Cu₂O.
Step 2: Determine the total mass of copper (I) oxide.
From the periodic table, the atomic mass of (Cu = 63.546, O = 15.999)
Cu₂O = (2 x 63.546) + 15.999
Cu₂O = 127.092 + 15.999 = 143.091
Step 3: Determine the percent composition of copper.
Mass of Cu in Cu₂O = 127.092
Therefore, the percent composition of Cu is:
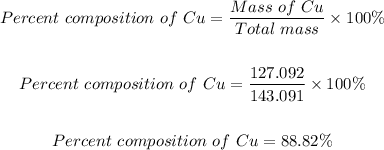
The percent composition of Cu = 88.82%
Step 4: Determine the percent composition of oxygen.
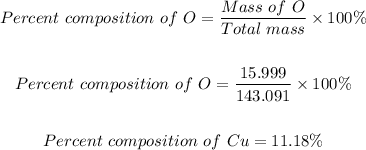
The percent composition of O = 11.18%