Solution:
Given that;
If the gas and piston has volume of 20.0L at a temperature of 25°C
To find the volume of the gas when heated tom 100°C, we will apply Charles law formula, which is

Where
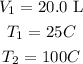
Substitute the values of the variables into the Charles law formula
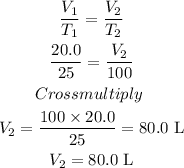
Hence, the answer is 80.0 L