First, find the total amount of money that they will pay.
Since each year has 12 months, then, there are 240 months in 20 years.
Since each month they pay $795.20, after 20 years they would have paid a total of:
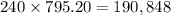
They have also paid $10,000 initially. Then, the total money spent in the house is:
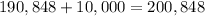
The value of the house is $80,000. Subtract $80,000 from the total amount of money that they have paid to find the total amount of interest:
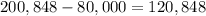
Therefore, the total amount of interest that the Blacks will pay is $120,848.