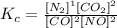The balanced equation of this reaction is

The equilibrium constant is given by the following.
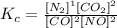
Observe that the exponents refer to the stoichiometry coefficients from the balanced equation, that's why we have to make sure the chemical equation is balanced before we find the expression of Kc. Additionally, Kc is equal to the ratio between the products and the reactants.
Therefore, the expression of the equilibrium constant is