the answer is 113.4 Kj
Step-by-step explanationHeat is referred to as a form of energy that can be easily achieved by burning fuel, the neat need can be calculated by using the formula:
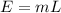
where E is the amount of heat(energy)
m is the mass
C is the specific heat capacity of water
T is the temperature
L is (specific heat of vapourisation of steam is 2268 kJ/kg)
Step 1
given
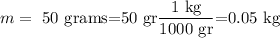
a)Heat released during conversion of steam at 100°C to water at 100°
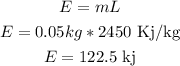
b)heat produced
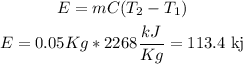
therefore, the answer is 113.4 Kj
I hope this helps you