So,
For this problem, we could identify the following values:
We know that the initial temperature is 77°F, which is 25°C.
The final temperature of water is 108°F, which is 42.22°C.
To convert °F to °C, we use:
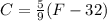
That's why 77°F equals 25°C, and 108°F equals 42.22°C.
The mass of water that we have equals 5.5 gal, which is 20.8197L of water. That's 20819.7g of water. Remember that 1 gal equals 4.5460 L, so, we just multiplied 5.5 gal by that amount to convert it to liters. Then, we divided by 1000 so we could get the amount in grams.
Now, the only thing we need to do is to replace all these values in the following equation:
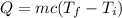
Where Q represents the energy required to raise this temperature to 108°F.
Given that c=1cal/g°C, we got:
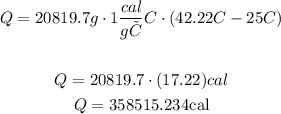
Now, these calories could be converted to Joules using the fact that 1 cal = 4.186 J, so:
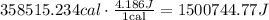
Now, we could use the fact that:
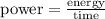
Where W is measured in J/sec.
Then,
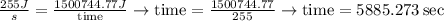
Finally, 5885.273 sec are about 98.1 minutes.
Therefore, it takes about 98 minutes to heat this amount of water from 77F to 108F.