Step-by-step explanation:
Given:
Molar mass of the compound = 133.60 g/mol
Volume of the solution = 471.4 mL
Molarity of the solution = 0.3 M.
The mass in grams of the compound = unknown
Note: Molarity (M) is defined as the number of moles of solute (n) divided by the volume (V) of the solution in liters, i.e
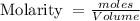
1 mL = 0.001 L
So, 471.4 mL will be 0.001 x 471.4 = 0.4714 L
Therefore, the mole of the compound can be calculated as follows:
![undefined]()