Answer:
16,066.56Joules
Explanations:
The amount of heat energy required is expressed according to the formula;
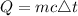
where:
• m is the mass of water = 48grams
,
• c is the specific heat capacity of water = 4.184 J/g°C
,
• △,t ,is the change in temperature, = 120 - 4,0 = 80°C
Substitute the given parameters into the formula
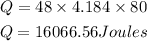
Hence the amount of energy required to heat 48 grams of water from 40 °C to 120 °C is 16,066.56Joules