The empirical formula is simply the ratio of each atom in the molecule.
The molecular formula is the actual number of each atom in one molecule.
To find it, we need to first find the mass of the empirical formula.
The empirical formula is C₃H₂O, so it has 3 carbon, 2 hydrogen and 1 oxygen.
The mass of these atoms are:
- Carbon: approximately 1.99 x 10⁻²⁰ mg
- Hydrogen: approximately 1.67 x 10⁻²⁰ mg
- Oxygen: approximately 2.66 x 10⁻²⁰ mg
So, the mass of the empirical formula is:
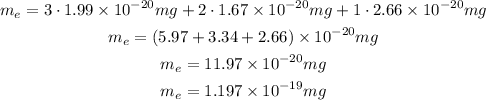
Now, we can see how many of this empirical formula we have in the molecular formula, so we divide the given mass of the molecule by the mass of the empirical mass:
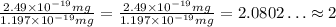
That is, the actual mass of the molecule is double the mass of the empirica formula, which means that we have twice as many of each atom in th molecular formula:
Molecular formula: (C₃H₂O)₂ -> C₆H₄O₂