Answer:
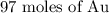
Step-by-step explanation:
Here, we want to get the number of moles in the given number of molecules of Au
Mathematically:
1 mole of a substance has 6.02 * 10^23 molecules
The number of moles in 5.82 * 10^25 molecules would be the division as follows:
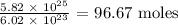
Approximately, there are 97 moles in the given number of molecules