Answer:
Step-by-step explanation:
Here, we want to get the new volume of the gas
What we need to know is the law that connects volume and pressure at constant temperature
The law that supports this is the Boyle's law
It states that the volume of a given mass of gas is inversely proportional to its pressure at constant temperature
Mathematically, we can have this as represented as:
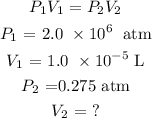
We can proceed mathematically to solve as follows;
![undefined]()