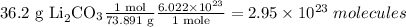Answer
2.95 x 102₃
Procedure
1. The first step is to search for the formula of lithium carbonate or deduct it using nomenclature rules,
-The formula is Li₂CO₃
2. Then determine the molecular weight of the molecule
-The molecular weight is 73.891 g/mol
3. Convert from grams to moles and from moles to molecules