Answer:
A. 8
Step-by-step explanation:
Point A is located at (-4,4).
If A is translated 6 units to the right and 5 units down, its image A' will be:
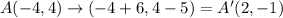
Next, find the distance between points A and A':
![\begin{gathered} Distance=√((x_2-x_1)^2+(y_2-y_1)^2) \\ =\sqrt[]{(2-(-4))^2+(-1-4)^2} \\ =\sqrt[]{(2+4)^2+(-5)^2} \\ =\sqrt[]{(6)^2+(-5)^2} \\ =\sqrt[]{36+25} \\ =\sqrt[]{61} \\ =7.81 \\ \approx8\text{ units} \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/8y2dmzcyh85jfyg4cwezsce6wwlbt8f3ue.png)
The distance between points A and A' is approximately 8 units.
The correct choice is A.