Answer
The mass (in grams) of oxygen present = 1.79 grams.
Step-by-step explanation
Given
Volume, V = 3.0 L
Temperature, T = 34 °C = (34 + 273.15 K) = 307.15 K
Pressure, P = 0.472 atm
What to find:
The mass (in grams) of oxygen present.
Step-by-step solution:
Step 1: Calculate the moles of oxygen present.
Using the ideal gas law:
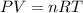
R is the molar gas constant = 0.0820574 L•atm/mol•K.
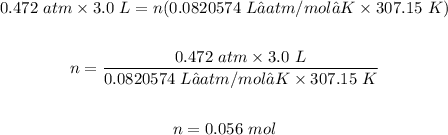
The moles of oxygen present is 0.056 mol.
Step 2: Convert 0.056 mol oxygen to mass in grams.
The molar mass of oxygen gas = 31.998 g/mol
Using the mole formula below, the mass of oxygen can be calculated as follows:
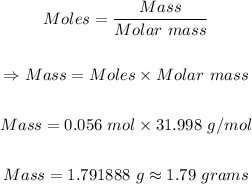
The mass (in grams) of oxygen present = 1.79 grams.