Answer:
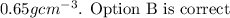
Explanations:
The formula for calculating the density of a substance is expressed as:
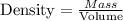
Given the following parameter:
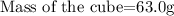
Get the volume of the cube:
![\begin{gathered} V=L^3 \\ V=4.6^3 \\ V=97.336\operatorname{cm}^3 \end{gathered}]()
Get the required density. Recall that;
![\begin{gathered} \text{Density of cube=}\frac{Mass\text{ of cube}}{Volume\text{ of cube}} \\ \text{Density of cube=}\frac{63.0g}{97.336\operatorname{cm}^3} \\ \text{Density of cube}\approx0.65\text{gcm}^(-3) \end{gathered}]()
Therefore the density of the material that composes the cube is 0.65g/cm^3