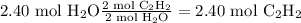Answer
2.40 mol C₂H₂
Procedure
The first step is to state the chemical reaction given
C₂H₂ + O₂ → CO₂ + H₂O
Then we will proceed to balance the equation by using the trial and error method
2C₂H₂ + 5O₂ → 4CO₂ + 2H₂O
After balancing the equation, we use the reaction stoichiometry to find the moles of acetylene needed to produce 2.40 moles of water, assuming oxygen is in excess.