Step 1 - Reading and understanding the chemical equation
The provided chemical equation is:
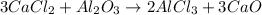
We can read this equation as follows:
three moles of CaCl2 react with one mole of Al2O3 thus producing two moles of AlCl3 and three moles of CaO
As the exercise is specifically asking about the proportion between Al2O3 and CaO, we can further simplify this statement to:
one mole of Al2O3 produces three moles of CaO
Step 2 - Converting the proportion in moles to a proportion in grams
To convert the number of moles to grams we just need to use the molar mass of each substance. (102 g/mol for Al2O3 and 56 g/mol for CaO)
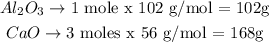
We can thus rewrite the proportion between these two substances as:
102g of Al2O3 produce 186g of CaO
Step 3 - Calculating how much CaO is produced
To calculate how much CaO is produced, we just need to set the following proportion:
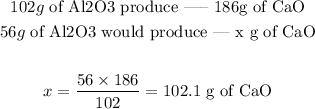
Answer: 102.1g of CaO would be produced