Given:
• Volume of air in lungs = 2.1 L
,
• Temperature of air = 37°C
Apply the equation of ideal gas law:

Rewrite the equation for n:
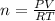
Where:
• P is the pressure = 1.013 x 10⁵ N/m²
,
• V is the volume in m³ = 2.10 x 10⁻³ m³
,
• R is the gas constant = 8.31 J/mol .K
,
• T is the temperature in kelvin
,
• n is the number of moles of atoms.
Now, convert the temperature to Kelvin:
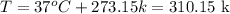
Now, substitute values into the equation and solve for n:
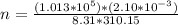
Solving further:
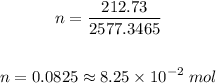
Therefore, the number of moles is 8.25 x 10⁻² mol
ANSWER:
8.25 x 10⁻² mol.