Given data
*The given internal energy is U = 140 J
*The given heat is Q = 60 J
The formula for the work can be done by the gas is given as
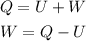
Substitute the known values in the above expression as
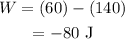
Here the negative sign indicates that the work can be done by the gas.