The reaction of combustion of octane is as follows:
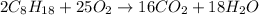
Convert the given mass of octane to moles using the molar mass of octane:
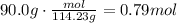
According to the given reaction, 2 moles of octane produce 18 moles of water. Use this ratio to find how many moles of water are produced from 0.79 moles of octane:
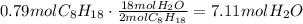
Multiply this amount times the efficiency of the reaction to find the actual amount of water produced:
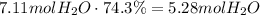
Convert this amount to moles using the molar mass of water:
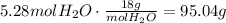
It means that the mass of water produced is 95.04g.