Answer:
606.81mL
Explanations:
To get the final volume of the gas sample, we will use Charles law. According to the law, the volume of a given amount of gas is directly proportional to its temperature. Mathematically;
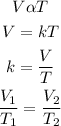
V1 and V2 are the initial and final volume
T1 and T2 are the initial and final temperatures.
Given the following parameters:
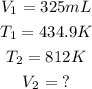
Substitute the given parameters into the formula to have:
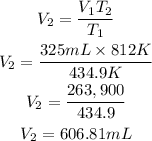
Hence the volume of the sample of gas if the temperature is increased to 812 K is 606.81mL