INFORMATION:
We have the next reaction:
- Mg(NO3)2 + Na2CO3
And we must find its net ionic equation
STEP BY STEP EXPLANATION:
To find the net ionic equation, we must follow the next steps
1. We must write the balanced equation
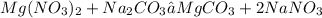
2. We must write the states
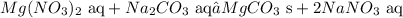
3. We must split strong electrolytes into ions
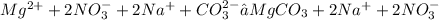
4. We must cross out the spectator ions on both sides
5. Finally, we must write the remaining substances
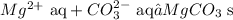
ANSWER:
The net ionic equation for Mg(NO3)2 + Na2CO3 is: