Answer:
6.17749 grams
Explanations:
The formula for calculating the mass of an element is expressed as:
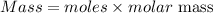
Moles of Lithium = 0.89moles
Molar mass of Lithium = 6.941g/mol
Determine the mass of lithium
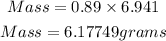
Hence the mass of the Lithium metal is 6.17749 grams