A change in temperature such as freezing point depression or boiling point elevation depends on three variables:
ΔT = k*m*i
ΔT is the change in temperature;
k is a constant that depends on the solvent;
and i depends on the number of particles that an ionic solute dissolves into
With this information, let's analyze each of the options, considering their dissolution in water:
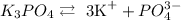
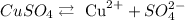

CH3OH is not ionic, so we do not consider any dissociation.
And finally,
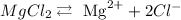
To know which will cause the least temperature change, we only count how many ions the compounds dissociate into.
However, the question is which will cause the least change, therefore it is the one that didn't have an ionic dissociation. CH3OH is the substance that will cause the least temperature change.