Oxidation-reduction reactions are those in which electrons are transferred from one chemical species to another, and the atom or ion that receives electrons has its charge or oxidation number (Nox) decreased, and we say that it has undergone a reduction. On the other hand, the species that loses electrons undergo oxidation, and its Nox increases.
Therefore, when balancing equations representing redox reactions, we aim to equalize the number of electrons that were lost and gained. And for that we first need to determine the Nox of all the elements of the substances in the reactants and products, and, with that, find out how many electrons were transferred and which species underwent reduction and oxidation.
The equation that we have is:
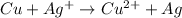
The oxidation number of
Cu: 0
Ag: +1
Cu: 2+
Ag: 0
Now let's determine the variation of Nox of each specie (it is the number of electrons transferred):
Cu: 2-0 = 2
Ag: 1-0 = 1
Cu oxidate, so it loses 2 electrons:
Cu --> Cu2+ + 2e
Cu suffer reduction, so it gain electrons:
Ag+ + 1e --> Ag
Ag have to win 2 electrons, because Cu loses 2, so let's multiply the equation by 2:
2 Ag+ + 2e --> 2Ag
Now we sum the two half-equations:
Cu --> Cu2+ + 2e
2 Ag+ + 2e --> 2Ag
__________________
Cu + 2Ag+ --> Cu2+ + 2Ag
Answer: Cu + 2Ag+ --> Cu2+ + 2Ag