Let's call A C10H8, B 12O2, C 10CO2, and D 4H2O.
The ratio is the division between two of the compounds.
For example, let's see the ratio between A and B:
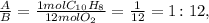
Now, let's see the ratio between A and C:
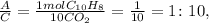
The mole ratio between B and C:
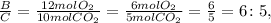
And finally, the mole ratio between B and D:
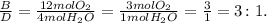
In this last example, you can interpret the mole ratios like this: three moles of O2 produce 1 mole of water (H2O). And so on with the other examples.