Firstly we will express ppb as mass per mass:
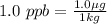
By using this definition we can determine the copper in the waste water sample:
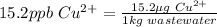
If 15.2 micrograms of copper is in 1 Kg of wastewater then in 1.23x10^3 kg of waste water we will have:
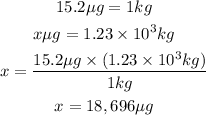
Answer: 18,696 micrograms of copper would be in 1.23x10^3kg of wastewater.