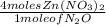Answer:
CORRECT
Explanations:
Given the balanced chemical reaction given as:
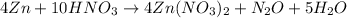
From the equation, 4 moles of Zn(NO3)2, also reacted with 1 mole of N2O. Hence the mole ratio from the equation will be the ratio of 4 moles of Zn(NO3)2 to 1 mole of N2O.
Hence the derived mole ratio as described is true.