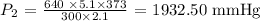Answer:

Step-by-step explanation:
Here, we want to calculate the final pressure
Mathematically from the general gas equation:
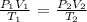
where:
P1 is the initial pressure which is 640 mmHg
V1 is the initial volume which is 5.1 L
T1 is the initial temperature (we convert this to Kelvin by adding 273: 27 + 273 = 300 K)
P2 is the final pressure which is what we want to calculate
V2 is the final volume which is 2.1 L
T2 is the final temperature (we convert to Kelvin by adding 273 K : 273 + 100 = 373 K)
Substituting the values, we have it that:
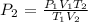
Substituting the values, we have it that: