Answer
No reaction.
Step-by-step explanation:
Given:
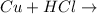
Copper,Cu cannot replace the hydrogen in HCl to form CuCl₂ because it is below hydrogen in the reactivity series. Hence, when copper (Cu) reacts with hydrochloric acid (HCl) there will be no reaction.