First, we have to find the number of moles of each element. The molar mass of phosphorus is 31 g/mol and the molar mass of bromine is 80 g/mol (You can find this in the periodic table):
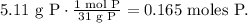
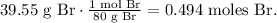
And then, we have to divide each value by the least value obtained. In this case, we have to divide everything by 0.165:
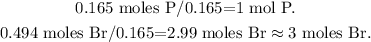
We're going to have 1 mol of phosphorus and 3 moles of bromine obtaining the empirical formula PBr3.