Answer:
8.33 moles of CO2 are produced.
Step-by-step explanation:
1st) It is necessary to balance the chemical equation:
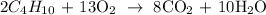
Now we know that 2 moles of C4H10 reacts with 13 moles of O2 to produce 8 moles of CO2 and 10 moles of H2O.
With the molar mass of C4H10 (58.12g/mol) and CO2 (44.01g/mol) we can convert moles into grams, so with 116.24g of C4H10 (2x58.12g) we obtain 352.08g of CO2 (8x44.01g).
2nd) Now we can calculate the moles of CO2 that will be produced from 121 grams of butane, using a mathematical rule of three:
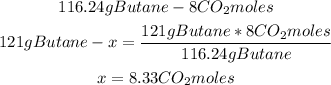
So, 8.33 moles of CO2 are produced.