Given,
The amount of concentration is 1.5 litre.
Number of capfuls used to make one litre of solution is 5.
The amount of concentration in each capfuls is 20 ml.
The number of capfuls made from 1.5 litre of concentration is,

There are 75 capfuls are made from the entire bottle of concentration.
The quantity of solution made from 75 capfuls is,
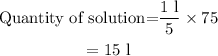
Hence, the solution can be made from the entire bottle of concentration is 15 litre.