Chemistry => Stoichiometry => Theoretical Yield and Percentage Yield
The percentage yield corresponds to the percentage that is actually obtained compared to what is theoretically expected.
We can define it by the following equation:
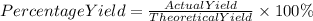
They already give us the theoretical and actual yield, so we can replace the known data in the equation.
Actual Yield = 1.72g of NH3
Theoretical Yield = 9.10g of NH3
So, the percentage yield will be:
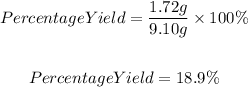
Therefore the answer will be: c. 18.9%