Solution
Given data
According to Boyle's law, PV = K
The required general equation thus is
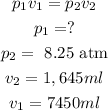
Substituting these values we find that the initial pressure is
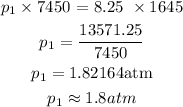
The initial pressure to the nearest tenth, therefore, is 1.8 atm.
The final answer, therefore, is Option D