Half life is the time that it takes for half of the original value of some amount of a radioactive element to decay.
We have the following equation representing the half-life decay:
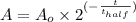
A is the resulting amount after t time
Ao is the initial amount = 50 mg
t= Elapsed time
t half is the half-life of the substance = 14.3 days
We replace the know values into the equation to have an exponential decay function for a 50mg sample
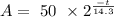
That would be the answer for a)
To know the P-32 remaining after 84 days we have to replace this value in the equation:
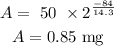
So, after 84 days the P-32 remaining will be 0.85 mg