We have according to the statement a gas to which external pressure is exerted to reduce its volume. We will first assume that the process occurs at a constant temperature and pressure. The work done by that external force is determined by the following form of work (W):
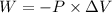
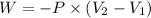
Where,
P is the external pressure, 1.15atm
V2 is the final volume, 3.16L
V1 is the initial volume, 6.55L
We replace the known values:
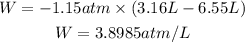
We will convert these units to joules. 1atm/L=101.325J.
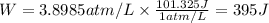
Answer: The work in joules done is 395 J