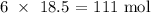Answer:
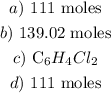
Step-by-step explanation:
a) From the balanced equation of reaction:
2 moles of dichlorobenzene produced 12 moles of carbon dioxide
18.5 moles of dichlorobenzene will produce x moles of carbon dioxide
To get the value of x, we have it that:
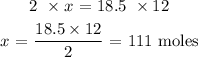
b) From the equation of reaction:
13 moles of oxygen produced 12 moles of carbon dioxide
150.6 moles of oxygen will produce x moles of carbon dioxide
To get the value of x, we have it that:
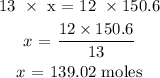
c) The limiting reagent would be the reactant that produces less of the product
dichlorobenzene produces less and thus, it is the limiting reactant
d) To get the theoretical yield, we multiply the number of moles of the limiting reagent by the ratio between the product and the limiting reagent
We have the number of moles of the limiting reagent by the mole ratio
We have the number of moles of the limiting reagent as 18.5 mol and the mole ratio as (product to reactant = 12:2 = 6 to 1)
We have the theoretical yield as: