The first step is to state the reaction between N2 and O2, which is:
Now, we have to compare how many grams of each reagent reacts. 2 moles of N2 react with one mole of O2, use the molecular weight of each reagent to know the amount of mass that reacts in the reaction:
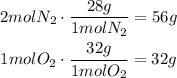
It means that the limiting reactant in this case is the N2. Use the ratio of grams to find how many gram of O2 react with 14.9 grams of N2:
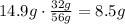
It means that 14.9g of N2 react with 8.5g of O2. To know how many grams of N2O are produced, find the sum of these masses:
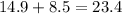
23.4g of N2O are produced.