Step 1
Gas here is assumed to be ideal. Then, the next equation is applied:
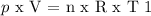
-----------------------------------------
Step 2
Information provided:
p = pressure = 3.25 atm
V = volume = 3.00 L
n = number of moles = unknown
T = absolute temperature = 300.0 K
R = gas constant according to the units = 0.082 atm L/mol K
-----------------------------------------
Step 3
n is found from (1):
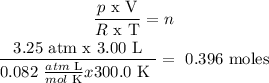
Answer: C) n = 0.396 moles