ANSWER
the volume of the solution is 2.05 liters
Step-by-step explanation
Given that;
The concentration of Ca(OH)2 is 0.420M
The grams of Ca(OH)2 is 64.0 grams
Follow the steps below to find the volume of the solution
Step 1; Calculate the number of moles of Ca(OH)2 using the below formula
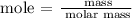
Recall, that the molar mass of Ca(OH)2 is 74.093 g/mol
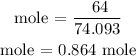
Step 2; Find the volume of the solution in liters using the below formula
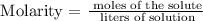
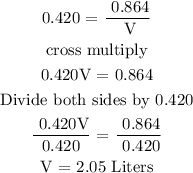
Therefore, the volume of the solution is 2.05 liters