Answer:
3.325 grams
Explanations:
The balanced chemical reaction for the decomposition of potassium chlorate is expressed as:
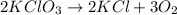
Determine the mole of potassium chlorate
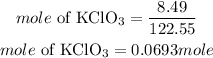
According to stoichiometry, 2 moles of potassium chlorate produces 3 moles of oxygen. The mole of oxygen required is expressed as:
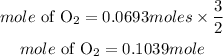
Calculate the required mass of oxygen
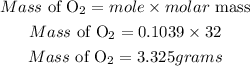
Hence the required mass of oxyeen required is 3.325 grams