Answer
The mass in grams of AgBr formed = 3.84 g
Step-by-step explanation
The given parameters are:
Volume of AgNO3 = 50.5 mL
Molarity of AgNO3 = 0.405 M
What to find:
The mass in grams of AgBr formed.
Step-by-step solution:
Step 1: Write a balanced equation for the reaction.
The balanced chemical equation for AgNO3 treated with an excess of aqueous hydrobromic acid is
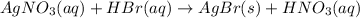
Step 2: Convert the volume of 0.405 M AgNO3 that reacted to moles.
The moles of AgNO3 that reacted can be determined using the molarity formula.
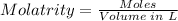
The volume of AgNO3 needs to be converted from mL to L using the conversion formula below.
Conversion factor: 1000 mL = 1 L
50.5 mL = (50.5 mL/1000 mL) x 1 L = 0.0505 L
So,
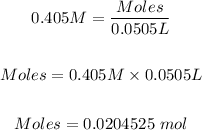
Step 3: calculated the moles of AgBr formed.
Using the mole ratio of AgNO3 and AgBr in step 1 and the moles of AgNO3 in step 2; the moles of AgBr is calculated as shown below.
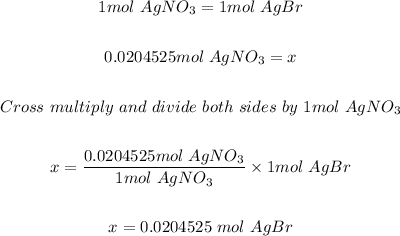
Step 4: Convert the moles of AgBr formed in step 3 to mass in grams.
Note that the molar mass of AgBr = 187.77 g/mol
Mass in grams of AgBr formed can be calculated using the mole formula.
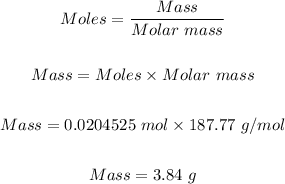
Therefore, the mass in grams of AgBr formed when 50.5 mL of 0.405 M AgNO3 is treated with an excess of aqueous hydrobromic acid is 3.84 g.