Answer:
The molarity of the solution is 0.99M.
Step-by-step explanation:
The molaruty if a solution represents the amount of solute moles contained in 1 liter (1000ml) of a solution.
1st) In this case, we have 17.3g of KCl, so we have to use the KCl molar mass (74.5g/mol) to convert grams into moles:
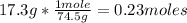
Now we know that there are 0.23 moles of the solute KCl.
2nd) We have 0.23 moles of KCl contained in 231 mL of solution, so to calculate the molarity (moles of solute contained in 1000mL of solution), we can use a mathematical rule of three:
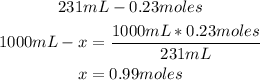
Finally, the molarity of the solution is 0.99M.