Given the following reaction:
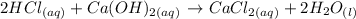
We want to know how many molecules of HCl will be needed to produce 4 molecules of H2O
We know that;
1 mole = 6.022x10^23 molecules
The mole ratio between HCl:H2O is 2:2, meaning we can use the stoichiometry, we know that the number of moles of HCl is the same for H2O.
If H2O has 4 molecules, lets first calcualate the number of moles.
1 mole = 6.022x10^23 molecules
x moles = 4 molecules
x = 4/6.022x10^23
x = 6.642x10^-24 moles of H2O
Since the molar ration of HCl:H2O is 2:2
Therefore the number of moles of HCl is 6.642x10^-24 moles
Now we can convert the number of moles to molecules.
number of molecules of HCl = 6.642x10^-24 x 6.022x10^23
= 4
There will be 4 HCl molecules needed.